Abstract
Introduction
6-mercaptopurine (6-MP) is a main component of childhood acute lymphoblastic leukemia (ALL) therapy. The sensitivity of 6-MP is associated with genetic variant of 6-MP metabolism. Recently, the NUDT15 genetic variant has been identified as a risk factor of 6-MP intolerability, and its association with 6-MP-induced toxicities and 6-MP dose in ALL patients have been reported. The frequency of NUDT15 hypomorphic variant is higher in Asian populations than in European and African populations. However, the 6-MP tolerable dose and efficacy for NUDT15-deficient patients remains clear. Our study aimed to evaluate 6-MP tolerable dose, the frequencies of 6-MP induced toxicities, and outcome in 17 ALL patients with NUDT15-deficient genotype.
Methods
We genotyped NUDT15 genetic variants and evaluated the patients with NUDT15 homozygous variant in Japanese childhood ALL. The NUDT15 variants V18_V19insGV, V18I, R139C, and R139H were genotyped by Sanger sequencing, and the diplotype was precisely determined. The standard initiation dose of maintenance therapy was 6-MP 40 to 50 mg/m2/day and methotrexate 25 mg/m2/week. The 6-MP-induced toxicities were graded by CTCAE version 4.0. The survival rate was estimated by the log-rank test.
Results
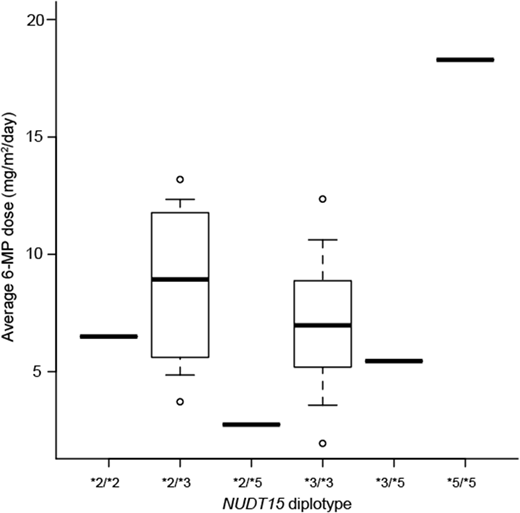
A total of 17 patients with NUDT15 diplotype of *2/*2, *2/*3, *2/*5, *3/*3, *3/*5, and *5/*5 were genotyped as NUDT15 deficient. Fifteen patients were B cell-precursor (BCP) ALL and 2 patients were T-ALL. Of the 15 BCP ALL patients, 13 were standard risk and 2 were high risk patients according to National Cancer Institute/Rome criteria. Grade 3 leukopenia and grade 4 neutropenia were observed in all 17 patients, and the median observation time were 33 (range 3-95) days and 35 (20-137) days after initiating maintenance therapy, respectively. Grade 3 ALT elevation was observed in 6 patients (35%), and median observation time was 47 (range 19-427) days after initiating maintenance therapy. Moreover, during the early consolidation phase with 6-MP, severe myelosuppression was observed in 11 of these patients. The average 6-MP dose during maintenance therapy was 7.0 (range 2.7-18.3) mg/m2/day. Moreover, 16 of these 17 patients (94%) with NUDT15 deficiency required median 66 (range 5-376) days of therapy interruption. Notably, the average 6-MP dose was 18.3 mg/m2/day, and no therapy interruption occurred during maintenance therapy in patients with NUDT15 *5/*5 diplotype. Therefore, the degree of NUDT15 deficiency influenced 6-MP tolerable dose.
The effect of NUDT15 deficiency on treatment outcome was evaluated in 14 patients, who completed treatment. Three patients relapsed at 124-388 days, and two of these three patients died at 877 and 959 days after the end of maintenance therapy, respectively. The overall and event-free survival rate at 4 years were 0.75 and 0.63, respectively. Neither the average 6-MP dose nor the interruption duration was associated with these events.
Conclusions
NUDT15-deficient genotypes strongly influence intolerability. Patients with NUDT15 deficiency did not tolerate standard 6-MP dose, and physicians should consider reducing 6-MP dose to 7 mg/m2 to avoid therapy interruption. Conversely, NUDT15 *5/*5 genotype displayed only mild NUDT15 deficiency, and the patients with this genotype tolerated 40% of the standard 6-MP dose. Further large-scale studies should be conducted to assess the NUDT15 variant's effect on survival.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal